Electronic Data Capture (EDC) has revolutionized the way clinical trials are conducted, streamlining data collection and analysis ...
- DCT
- ProductsCLINICAL DATA MANAGEMENT

EDC
One size does not fit all

RTSM
Deliver powerful randomization methods

CODER
Fast and accurate clinical coding

Drug and Device Logistics
Mitigate risk and create closer connections
CLINICAL OPERATIONS
eTMF
Essential document collection

CTMS
Accelerate your trial and ensure data integrity
PATIENT CENTRICITY
eCONSENT
Meet patients where they are

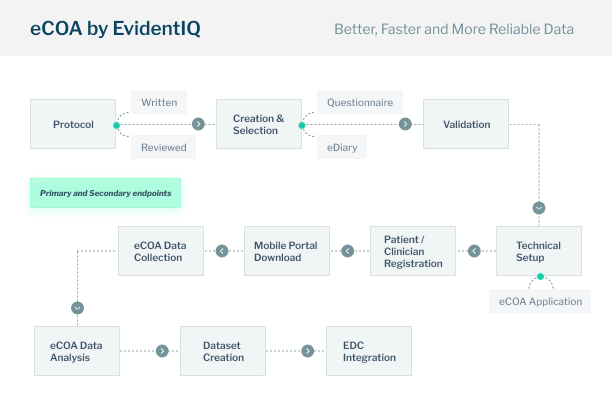
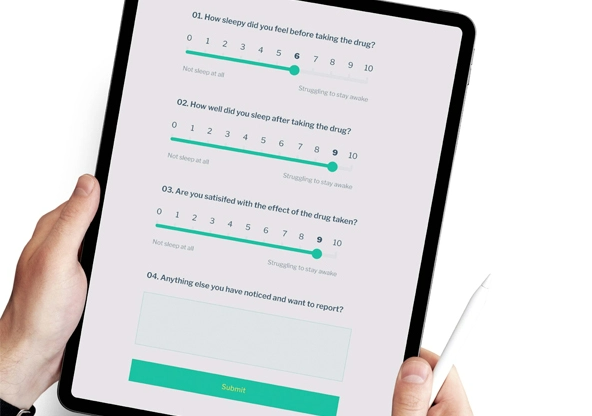
eCOA
Faster, cleaner and more reliable data

Patient Poll
Quick insights from patients
CLINICAL DATA MANAGEMENTPATIENT CENTRICITYCLINICAL OPERATIONS - ServicesCLINICAL TRIAL SERVICES

eCRF Setup
Capture better data, faster

eTMF Setup
Get up and running in no time

PV Management
Identify and handle your safety management needs

Data Management
Make more informed business decisions

Managed Service Model for MDR
Migration, Consulting and Customization for your MDR

Submission and Data Mapping
Move and consolidate data

eTechnology Strategy
Integrate and capitalize on critical technologies

Protocol Development
Turning your research question into a study
DATA SCIENCE SERVICES
Observational Studies
Digital, cost effective and time efficient

Primary Market Research
Direct to patient, unique, and quality data

IRB and EC Management
End to end regulatory management

PV and GDPR Compliance
Safety, consent and data protection

Scientific Communications
Results dissemination

Digital Communications
Increase patient awareness

Data Linkage
Generate, combine and analyze data
CLINICAL TRIAL SERVICESDATA SCIENCE SERVICES - Patients
- Resources
- Company